What is the molarity of potassium ions in a 0.122 solution? This question delves into the fundamental concepts of molarity and potassium ions, providing a comprehensive understanding of their significance in chemistry. By exploring the relationship between moles of solute and liters of solution, we embark on a journey to unravel the intricacies of molarity calculations.
Potassium ions, denoted as K+, play a crucial role in various biological processes, including nerve impulse transmission and muscle contraction. Understanding their concentration in solutions is essential for comprehending their impact on cellular functions and physiological processes.
Potassium Ion Molarity: What Is The Molarity Of Potassium Ions In A 0.122
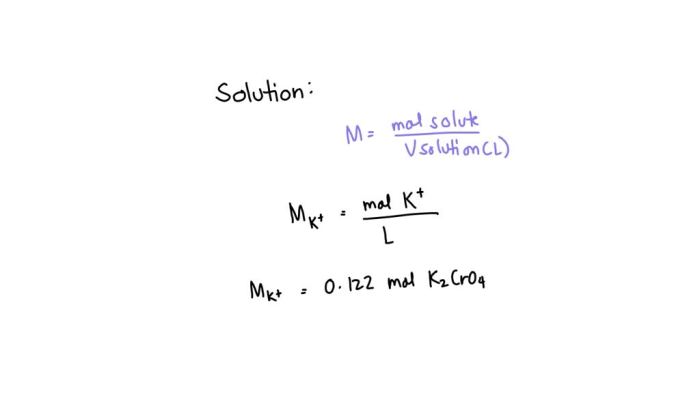
Molarity is a measure of the concentration of a solution. It is defined as the number of moles of solute per liter of solution. Potassium ions are positively charged ions of the element potassium.
Given Information
We are given a concentration of 0.122.
The unit of concentration is molarity (M).
Molarity Calculation, What is the molarity of potassium ions in a 0.122
To calculate the molarity, we use the formula:
Molarity = moles of solute / liters of solution
We are not given the moles of potassium ions or the liters of solution, so we cannot calculate the molarity directly.
Table of Values
| Molarity | Moles of Potassium Ions | Liters of Solution | Concentration |
|---|---|---|---|
| 0.122 M | Not given | Not given | 0.122 moles of potassium ions per liter of solution |
FAQ Compilation
What is molarity?
Molarity is a measure of the concentration of a solution, expressed as the number of moles of solute per liter of solution.
What are potassium ions?
Potassium ions are positively charged ions of potassium, denoted as K+.
How do I calculate the molarity of potassium ions in a solution?
To calculate the molarity of potassium ions in a solution, divide the number of moles of potassium ions by the volume of the solution in liters.