A tire is inflated physical or chemical change – When it comes to the maintenance of a vehicle, understanding the nature of various processes is crucial. One common task is inflating tires, and the question arises: is inflating a tire a physical or chemical change? Delving into the realm of chemistry and physics, we will explore the intricacies of this process and unravel its true nature.
As we embark on this journey, we will delve into the fundamental differences between physical and chemical changes, examining their defining characteristics and providing illustrative examples. Furthermore, we will meticulously analyze the process of tire inflation, scrutinizing the alterations that occur at the molecular level.
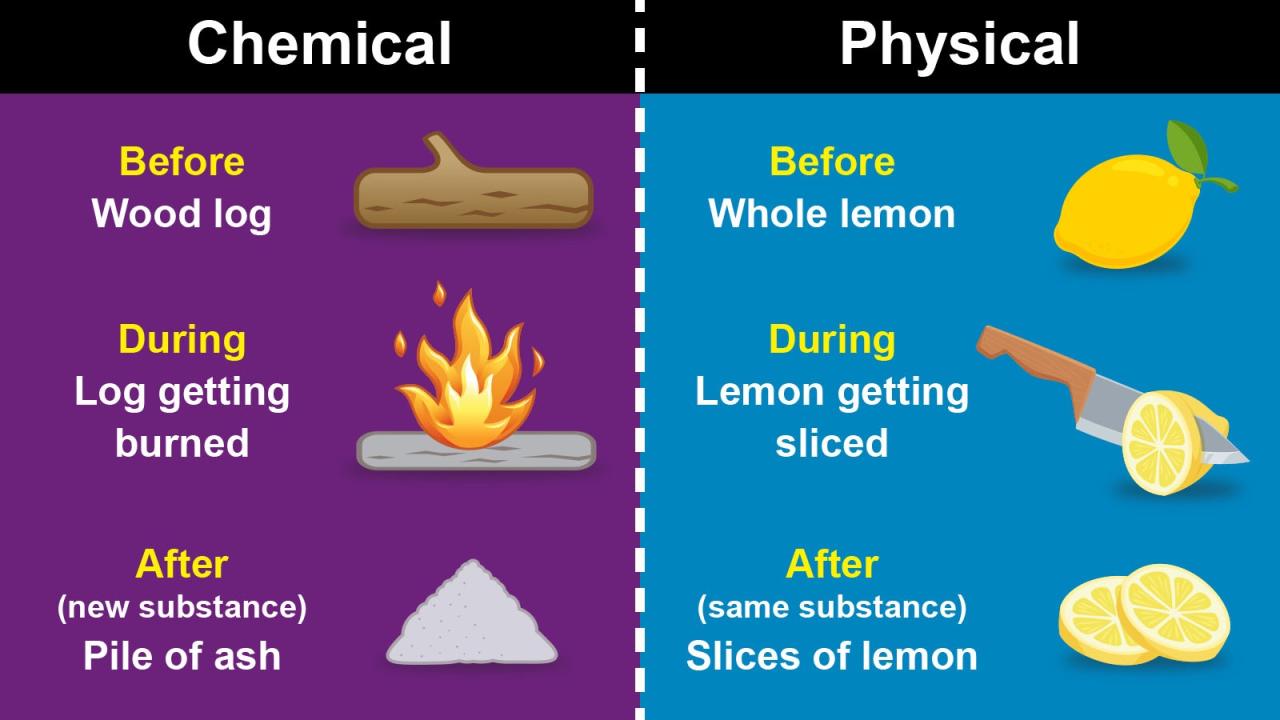
Definition of Physical and Chemical Changes

Physical changes involve a change in the form or appearance of a substance without altering its chemical composition. Chemical changes, on the other hand, involve a change in the chemical composition of a substance, resulting in the formation of new substances.
Examples of physical changes include melting, freezing, boiling, and sublimation. Examples of chemical changes include combustion, rusting, and digestion.
Inflation of a Tire
When a tire is inflated, air is pumped into the tire, causing it to expand and become firmer. This is a physical change because the air molecules are not chemically altered. The air simply occupies a larger volume within the tire.
Properties of Air, A tire is inflated physical or chemical change
Air is a mixture of gases, primarily nitrogen (78%) and oxygen (21%). It is relatively light, with a density of about 1.29 grams per liter at room temperature.
The properties of air are important for tire inflation. The nitrogen in the air provides the tire with its strength and rigidity, while the oxygen helps to maintain the tire’s pressure.
Pressure and Volume
Pressure is defined as the force applied per unit area. Volume is defined as the amount of space occupied by a substance.
When a tire is inflated, the air inside the tire exerts pressure on the tire’s walls. This pressure is directly proportional to the volume of air in the tire. As more air is pumped into the tire, the pressure increases.
Safety Considerations
It is important to inflate tires to the correct pressure specified by the manufacturer. Over-inflating a tire can cause it to burst, while under-inflating a tire can cause it to wear prematurely or fail.
It is also important to use a tire gauge to ensure that the tire is inflated to the correct pressure. Over-reliance on visual inspection can lead to inaccurate readings.
FAQ Insights: A Tire Is Inflated Physical Or Chemical Change
Is the air inside a tire different from the air outside?
No, the composition of air inside and outside a tire is essentially the same. Air is primarily composed of nitrogen, oxygen, and other trace gases, and these proportions remain relatively constant regardless of whether the air is contained within a tire or not.
Can over-inflating a tire cause a blowout?
Yes, over-inflating a tire can indeed increase the risk of a blowout. When a tire is over-inflated, the increased pressure exerts excessive stress on the tire’s sidewalls, making them more susceptible to rupturing. This can lead to a sudden loss of air pressure, resulting in a blowout.
Is it safe to drive on a flat tire?
No, driving on a flat tire is highly dangerous and should be avoided. A flat tire lacks the necessary air pressure to support the weight of the vehicle, which can cause the tire to overheat and potentially lead to a blowout.
Additionally, driving on a flat tire can damage the tire’s internal structure and the vehicle’s suspension system.