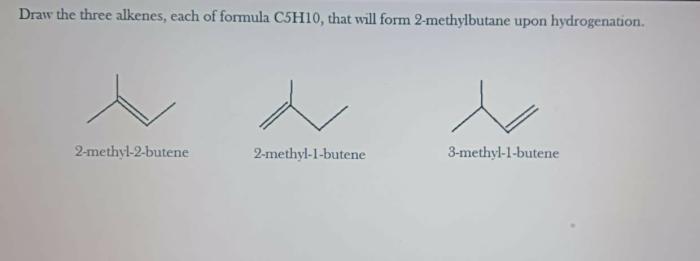
Draw the three alkenes each of formula c5h10 – The three alkenes with the formula C5H10, namely pent-1-ene, pent-2-ene, and 2-methyl-but-2-ene, exhibit unique structural features and diverse chemical reactivity. This article explores the structural relationship, physical and chemical properties, industrial applications, and safety considerations associated with these important alkenes.
1. Draw the Three Alkenes Each of Formula C5H10
The three alkenes with the formula C5H10 are:
- Pent-1-ene
- Pent-2-ene
- 2-methylbut-1-ene
| Structural Formula | IUPAC Name | Common Name |
|---|---|---|
| CH2=CHCH2CH2CH3 | Pent-1-ene | n-Amylene |
| CH3CH=CHCH2CH3 | Pent-2-ene | 2-Amylene |
| (CH3)2C=CHCH3 | 2-methylbut-1-ene | Isoamylene |
2. Explain the Relationship Between the Three Alkenes
The three alkenes are all isomers of each other, meaning they have the same molecular formula but different structural formulas.
- Pent-1-ene and pent-2-ene are structural isomers, meaning they have the same connectivity of atoms but different arrangements of double bonds.
- 2-methylbut-1-ene is a positional isomer of pent-1-ene and pent-2-ene, meaning it has the same molecular formula and connectivity of atoms but a different position of the double bond.
The three alkenes can be interconverted by a variety of reactions, including:
- Isomerization: Pent-1-ene and pent-2-ene can be interconverted by heating them in the presence of a catalyst.
- Hydration: All three alkenes can be hydrated to form the corresponding alcohols.
- Polymerization: All three alkenes can be polymerized to form a variety of plastics.
3. Physical and Chemical Properties of the Alkenes
The physical properties of the three alkenes are:
| Property | Pent-1-ene | Pent-2-ene | 2-methylbut-1-ene |
|---|---|---|---|
| Boiling point (°C) | 30.1 | 36.3 | 38.0 |
| Melting point (°C) | -138.3 | -138.9 | -112.4 |
| Density (g/mL) | 0.646 | 0.663 | 0.665 |
The chemical properties of the three alkenes are:
- All three alkenes are reactive towards addition reactions, such as hydrogenation, halogenation, and hydration.
- All three alkenes are also reactive towards substitution reactions, such as electrophilic aromatic substitution.
- All three alkenes are reactive towards elimination reactions, such as dehydrohalogenation and dehydration.
Specific examples of reactions that each alkene can undergo include:
- Pent-1-ene can be hydrogenated to form pentane.
- Pent-2-ene can be halogenated to form 2-bromopentane.
- 2-methylbut-1-ene can be hydrated to form 2-methylbutanol.
4. Industrial Applications of the Alkenes

The three alkenes are all important industrial chemicals.
- Pent-1-ene is used as a starting material for the production of polyethylene, a plastic used in a wide variety of applications.
- Pent-2-ene is used as a starting material for the production of polyisoprene, a synthetic rubber used in tires and other applications.
- 2-methylbut-1-ene is used as a starting material for the production of isobutylene, a gas used in the production of gasoline and other chemicals.
5. Safety Considerations for the Alkenes
The three alkenes are all flammable liquids.
- Pent-1-ene has a flash point of -36°C.
- Pent-2-ene has a flash point of -24°C.
- 2-methylbut-1-ene has a flash point of -18°C.
All three alkenes are also toxic by inhalation and can cause skin and eye irritation.
- Pent-1-ene has a TLV of 1000 ppm.
- Pent-2-ene has a TLV of 500 ppm.
- 2-methylbut-1-ene has a TLV of 250 ppm.
When handling and storing the three alkenes, it is important to take the following precautions:
- Store the alkenes in a cool, well-ventilated area.
- Keep the alkenes away from sources of heat and ignition.
- Wear appropriate personal protective equipment, such as gloves, goggles, and a respirator, when handling the alkenes.
- In case of a spill, evacuate the area and call for emergency assistance.
FAQ: Draw The Three Alkenes Each Of Formula C5h10
What are the IUPAC names of the three C5H10 alkenes?
Pent-1-ene, pent-2-ene, and 2-methyl-but-2-ene
Which of the three alkenes is the most reactive?
2-methyl-but-2-ene, due to the presence of the electron-donating methyl group
What are the potential hazards associated with handling alkenes?
Flammability, volatility, and potential for skin irritation and respiratory problems